The G.E. reagent is 3:1 acetic acid to glycerol
24 July 2023
Recently I've been trying microcrystallization, a
technique for identifying lichen chemicals, where extracts
of lichen chemicals are crystallized using various
reagents. Once applied, the crystals are allowed to
form (think a "crystal growing kit" from your childhood,
but much smaller!) and then are viewed under high powered
microscope (usually 100-400x).
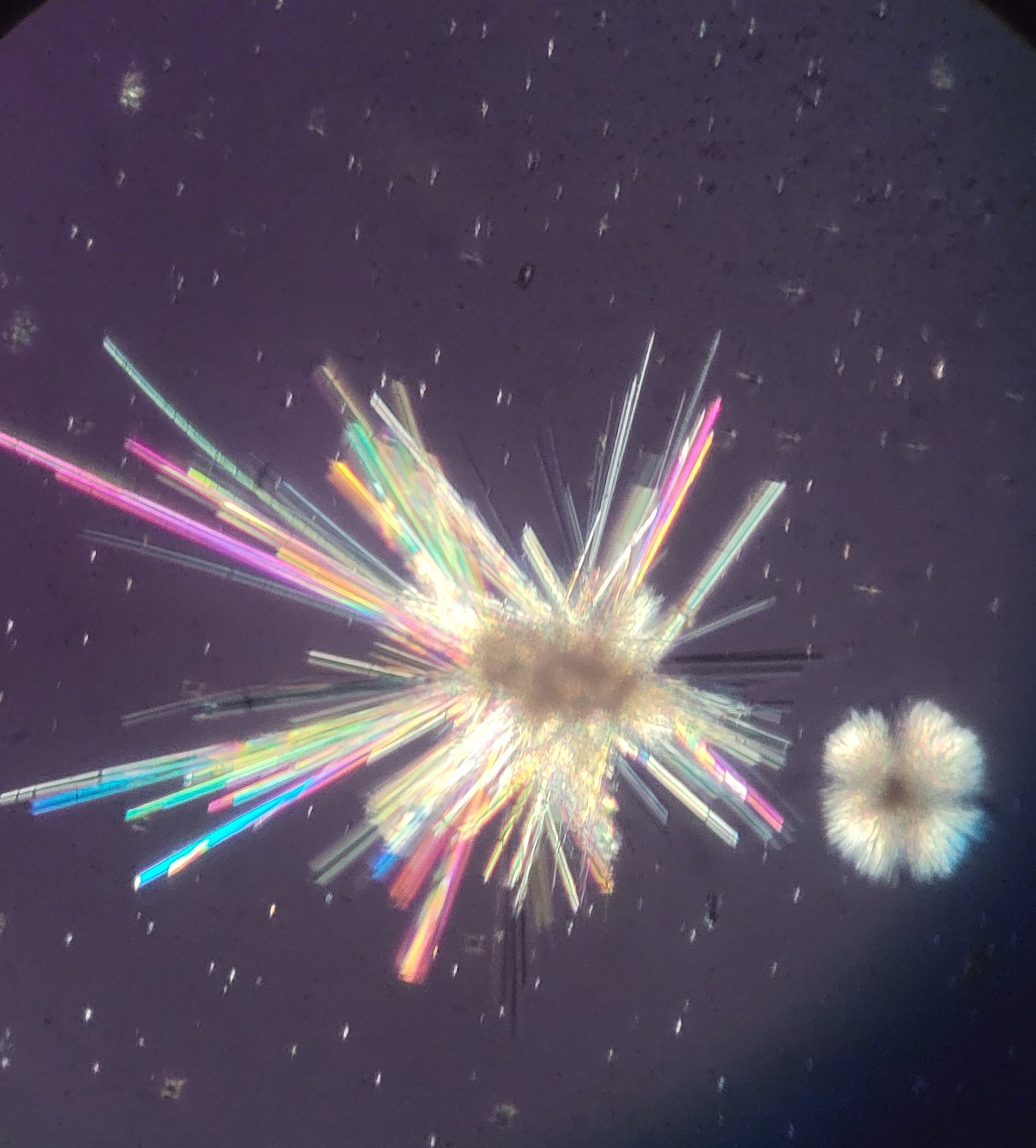
3:1 or 1:3?
One of the most common reagents is G.E., which is made up
of glycerol (a.k.a. glycerin) and glacial acetic acid.
When reading about the G.E. reagent I noticed a confusing
discrepancy; some sources gave it as 3:1 glycerol:acetic
acid, and some as 3:1 acetic acid:glycerol. After some
searching, the earliest reference to the G.E. reagent I
could find was in a paper by Yasuhiko Asahina, who
pioneered the technique in general, in 1936
(Mikrochemischer Nachweis der Flechtenstoffe
II). This paper says G.E. is 3:1 acetic
acid:glycerol, which, as far as I can tell, is the
correct formula, and the one lichenologists used.

I believe the 1996 book Identification of Lichen Substances by Siegfried Huneck and Isao Yoshimura to be the earliest example of this error. Another book with the error, from 2001, Microchemical Methods for the Identification of Lichens by A. Orange, P.W. James and F.J. White, cites Huneck. It feels like an easy mistake to make; G.E. is intuitively mostly the G (glycerol), not the E ("ethanoic acid", the systematic name for acetic acid).
Tragically, Huneck's book has a large number of photos of microcrystallization, most of which using G.E. The formula for G.E. is only given once in the book, so it's unclear whether they followed it when taking the photos, or if it was just a typo.
Why it matters
Microcrystallization as a technique has long since fallen out of favor due to thin-layer chromatography, another analytical technique capable of identifying small amounts of substances and requiring less subjective interpretation of results. However, unlike thin-layer chromatography, microcrystallization is easy to do, requires less hands on time, and doesn't require the use of dangerous chemicals.
For these reasons, I believe microcrystallization could have a place for separating species which differ only in chemistry. However, without a high quality and reliable source of images to compare with, microcrystallization might fall out of favor.
Although the error seems limited to these books, they are both frequently referenced (Google Scholar says 1,277 citations of Huneck and 2,262 citations for Orange). It's troubling to think that readers may reach for one of these books when preparing G.E., with confusing results when their crystals don't form as expected, or, if they do catch the error, that they might be discouraged by the conflicting information. Getting to the bottom of the issue for me required tracking down the Asahina article, which is in German and from 1936.
I want to help this situation by providing resources about microcrystallization online. Although Huneck is immensely valuable, the photos often lack the lichen source, as well as when the photos were taken relative to applying the reagent (some crystals form very slowly, some very quickly). A way of querying photos of microcrystallization results by taxa, reagent, extraction method, etc. would do a lot for the usefulness of the technique.
References and resources
-
Ohmura, Y.
Microcrystal Tests for Lichen Substances/地衣類化学成分の顕微結晶法 [Video]
https://www.youtube.com/watch?v=2IoUzuuf8Ww
(2022).
(Includes a video at the end of performing microcrystallization. It cites the correct G.E. formula.) -
Orange, A.; James, P.W.; White, F.J.
Microchemical Methods for the Identification of
Lichens (2001).
(This book is a guide for a vast array of analytical techniques and is frequently cited for its description of thin layer chromatography. It has a complete guide on microcrystallization, but doesn't include many photos as reference.) -
Huneck, S.; Yoshimura, I.
Identification of Lichen Substances
(1996).
(To my knowledge, one of the largest sources of microcrystallization images. Unfortunately has the wrong G.E. formula.)